有关明日叶植物的医学用途及药理的综述
Georg Thieme Verlag KG Stuttgart · New York
A Review of the Medicinal Uses and Pharmacology of Ashitaba
Lindsay K. Caesar, Nadja B. CechDepartment of Chemistry and Biochemistry, University of North Carolina at Greensboro, Greensboro, NC, USA
【中文摘要】
格奥尔格,斯图加特,纽约
有关明日叶植物的医学用途及药理的综述
*AD:关于明日叶,免费咨询更多,添加微信:156440577
林赛 k. 凯撒,娜迪亚 b. 切赫
化学与生物系,格林斯博罗北卡罗莱纳大学,格林斯博罗,美国
摘 要
明日叶在日本是一种很受欢迎的植物性药材,含有多种不同的生物活性成分,包括异戊烯基查尔酮、线型和体型结构香豆素、黄烷酮类等。
本文概述了明日叶代谢产物及其生物活性的最新知识来促进未来的研究。明日叶据称可以控制细胞毒素,具有抗糖尿病、抗氧化、抗炎、抗高血压和抗菌的特性。
尽管已经进行了很多对明日叶的化学成分的体外研究,但这种植物的体内疗效和临床相关性还有待证实。
在这里,以现有文献作为支持,我们描述了明日叶的化学成分,并介绍了该植物性药材的药理学功效。这些实验结果展示了明日叶医疗用途的可能性,但是还需要相当大量的工作来解析其代谢产物的活动机制。此外,体内和临床试验以及更多更丰富的生物活性化合物的研究是必要的。
*AD:免费领取明日叶30粒、茶包体验装,添加微信:156440577
关键字:明日叶、伞形科、生物活性研究、香豆素、异戊烯基查尔酮
【英文论文原文】
Abstract
(摘要)
Angelica keiskei Koidzumi, or ashitaba, is a popular botanical medicine in Japan containing diverse bioactive components including prenylated chalcones, linear and angular coumarins, and flavanones. This review provides an overview of the current knowledge of ashitaba metabolites and their biological activities to prioritize future studies. Ashitaba is purported to possess cytotoxic, antidiabetic, antioxidative, anti-inflammatory, antihypertensive, and antimicrobial properties. Although many in vitro studies have been conducted on ashitabaʼs chemical constituents, the in vivo efficacy and clinical relevance of this plant has yet to be confirmed for most of these activities. Here we describe the chemical composition of ashitaba and present the pharmacological effects of this botanical as supported by the current literature. The experimental results demonstrate promise for the medical use of ashitaba, but considerable work needs to be done to understand the mechanisms of action of its metabolites. Additionally, in vivo and clinical trials as well as additional studies on less abundant bioactive compounds are warranted.
Key words:Angelica keiskei – Apiaceae – bioactivity studies – coumarins – prenylated chalcones
Introduction
(前言)
Medicinal plants are commonly employed for therapeutic purposes throughout the world. A recent National Health Interview Survey estimated that nearly 18 % of adults in the United States regularly took non-vitamin, non-mineral dietary supplements in 2012 [1]. Because of the popularity of herbal medicines, it is important to understand the chemical basis behind the purported activities of botanicals. Angelica, a member of the Apiaceae (Umbelliferae) family, is a large genus comprised of over 60 species. Members of the genus have been utilized as medicines across the world, most notably in Asia, to treat numerous ailments, including influenza, hepatitis, arthritis, indigestion, fever, and microbial infections [2]. An increasing number of studies are being conducted on a medicinally promising member of the genus, Angelica keiskei Koidzumi (Apiaceae), or ashitaba. This large leafy perennial plant native to the Pacific coast of Japan is used throughout Asia for its diuretic, laxative, stimulant, and galactagogue properties [3]. In the past decade, several active constituents representing chalcones, flavanones, and coumarins have been isolated and characterized from ashitaba, and several bioactivities have been described. This review presents the current progress on ashitaba pharmacological studies, with a focus on isolated secondary metabolites, biological activity, toxicological data, and clinical relevance.
Bioactive Metabolites Isolated from Ashitaba
(明日叶分离出的生物活性物质)
*AD:选购明日叶种子、种苗等,基地直发,添加微信:156440577
Chalcones(芳丙烯酰芳烃/查尔酮)
Most of the literature on the bioactive metabolites from ashitaba concerns the diverse activity of various chalcones ([Table 1] and [Fig. 1]), which are most abundant in the root bark of the plant [4]. Chalcones are formed from phenylpropanoid starter units, extended with three malonyl-CoA molecules. The resulting polyketide is folded by the enzyme chalcone synthase to promote Claisen condensations and subsequent enolizations [5]. Interestingly, the bioactive chalcones found in ashitaba are prenylated at the 5′-position ([Fig. 1]), indicating that these molecules have undergone multiple biosynthetic steps, travelling through the acetate, shikimate, and isoprenoid pathways.
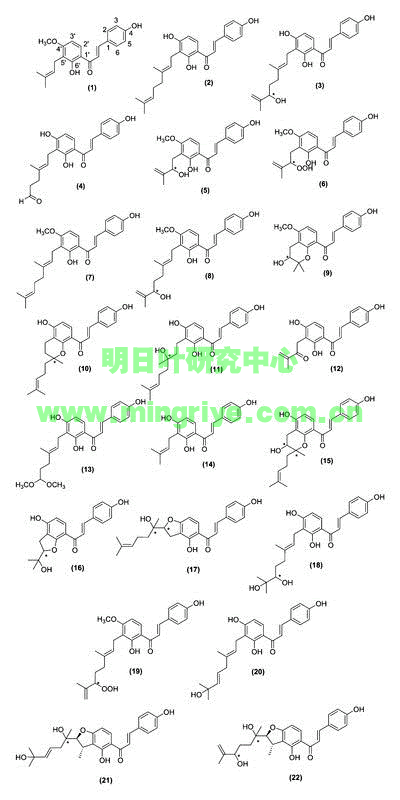
Fig. 1 Structures of bioactive chalcones isolated from A. keiskei Koidzumi. Absolute configuration at points marked with an asterisk (*) were not specified in original articles.
Table 1
Isolated bioactive components from A. keiskei Koidzumi and part of the plant from which they were first isolated.
| No. | Compound name | Part of plant | References |
| aPart of plant was inferred, but not directly stated by authors. bCommon names laserpitin and isolaserpitin also refer to sesquiterpene-type compounds. In this case, they refer to angular coumarin derivatives isolated from Ashitaba fruits. Other references cited in this review utilize this nomenclature as well | |||
| Chalcones | |||
| 1 | 4-hydroxyderricin | Roots | [50] |
| 2 | xanthoangelol | Roots | [50] |
| 3 | xanthoangelol B | Roots | [51] |
| 4 | xanthoangelol C | Roots | [51] |
| 5 | xanthoangelol D | Roots | [51] |
| 6 | xanthoangelol E | Roots | [51] |
| 7 | xanthoangelol F | Roots | [52] |
| 8 | xanthoangelol G | Roots | [52] |
| 9 | xanthoangelol H | Roots | [52] |
| 10 | xanthoangelol I | Stems | [3] |
| 11 | xanthoangelol J | Stems | [3] |
| 12 | xanthoangelol K | Stems | [18] |
| 13 | xanthokeistal A | Leaves a | [39] |
| 14 | isobavachalcone | Roots | [52] |
| 15 | (2E)-1-[3,5-dihydroxy-2-methyl-2-(4-methyl-3-penten-1-yl)-3,4-dihydro-2H-chromen-8-yl]-3-(4-hydroxyphenyl)-2-propen-1-one | Roots | [27] |
| 16 | (2E)-1-[4-hydroxy-2-(2-hydroxy-2-propanyl)-2,3-dihydro-1-benzofuran-7-yl]-3-(4-hydroxyphenyl)-2-propen-1-one | Roots | [27] |
| 17 | (2E)-1-[4-hydroxy-2-(2-hydroxy-6-methyl-5-hepten-2-yl)-2,3-dihydro-1-benzofuran-5-yl]-3-(4-hydroxyphenyl)-2-propen-1-one | Roots | [27] |
| 18 | (2E)-1-(3-[(2E)-6,7-dihydroxy-3,7-dimethyl-2-octen-1-yl]-2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one | Roots | [27] |
| 19 | (2E)-1-(3-[(2E)-6-hydroperoxy-3,7-dimethyl-2,7-octadien-1-yl]-2-hydroxy-4-methoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one | Roots | [27] |
| 20 | xanthokeismin A | Stems | [31] |
| 21 | xanthokeismin B | Stems | [31] |
| 22 | xanthokeismin C | Stems | [31] |
| Coumarins | |||
| 23 | (3′R)-3′-hydroxy-columbianidin | Stems | [17] |
| 24 | 3′-senecioyl khellactone | Stems | [17] |
| 25 | 5-methoxypsoralen | Fruit | [53] |
| 26 | 4′-senecioyl khellactone | Stems | [17] |
| 27 | archangelicin | Fruit | [53] |
| 28 | isolaserpitin b | Fruit | [53] |
| 29 | laserpitin b | Fruit | [53] |
| 30 | osthenol | Stems | [3] |
| 31 32 33 |
pteryxin demethylsuberosin selinidin |
Stems Aerial portion Fruit |
[17] [24] [53] |
| Flavanones | |||
| 34 35 |
8-geranylnaringenin 4′-O-geranylnaringenin |
Stems Stems |
[3] [17] |
| 36 | isobavachin | Stems | [3] |
| 37 38 |
munduleaflavanone munduleaflavanone B |
Stems Stems |
[17] [3] |
| 39 | prostratol F | Stems | [17] |
| Other compounds | |||
| 40 | ashitabaol A | Seeds | [22] |
| 41 | falcarindiol | Stems | [17] |
| 42 | pregnenolone | Aerial portion | [24] |
| 43 | 4-hydroxy-3,5,5-trimethyl-4-(1,2,3,-trihydroxybutyl)cyclohex-2-enone | ||
Ashitaba contains numerous coumarins with medicinal properties ([Table 1] and [Fig. 2]). Coumarins result from the addition of a hydroxy group, ortho- or para-, to the propanoid side chain of cinnamic acids [13]. Although basic coumarins are comprised solely of a phenylpropanoid backbone with varying degrees of hydroxylation, many others have more complex carbon frameworks derived from isoprene units. These 5-carbon units can lead to cyclization with a phenol group, eventually yielding complex coumarin derivatives [13]. Depending on the position of the initial dimethylallylation, furocoumarin derivatives may be angular (23, 24, 26–29, 31, 33) or linear (25).Coumarins(香豆素类)
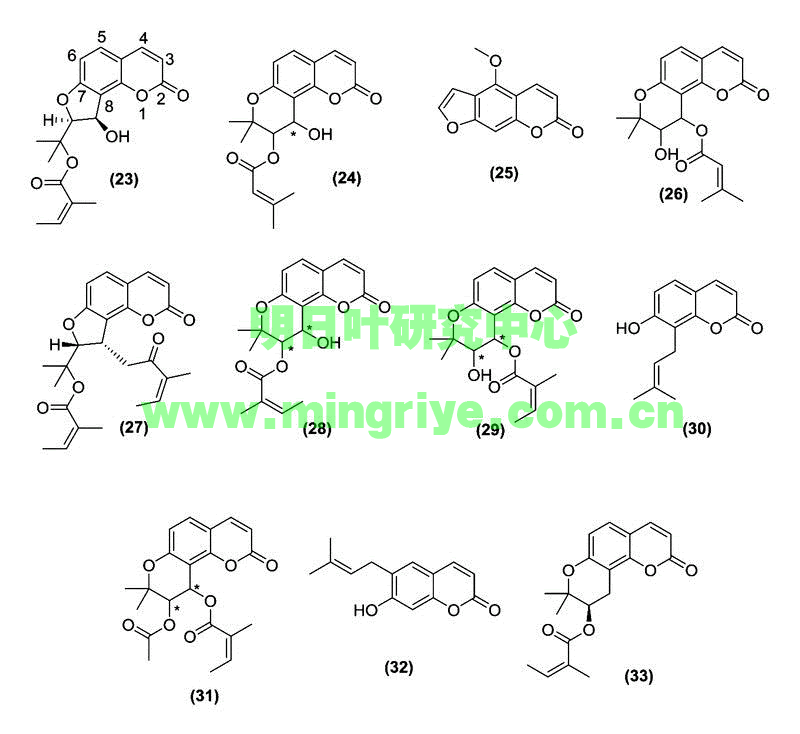
Fig. 2 Structures of bioactive coumarins isolated from A. keiskeiKoidzumi. Absolute configuration at points marked with an asterisk (*) were not specified in original articles.
Coumarins isolated from a number of plant species have been shown to possess anti-inflammatory and chemopreventive properties [14], [15]. Indeed, coumarins isolated from ashitaba have demonstrated cytotoxic properties [3], [16], [17] in addition to antidiabetic [18], antiobesity [12], and blood pressure-reducing effects [19].
Flavanones(黃烷酮类)
Considering the abundance of chalcones found in ashitaba, it is not surprising that this plant also possesses several flavanones ([Table 1] and [Fig. 3]). Chalcones, with a nucleophilic phenol group positioned near to an α,β-unsaturated ketone, readily undergo a Michael-type attack, leading to cyclization and flavanone formation [20].
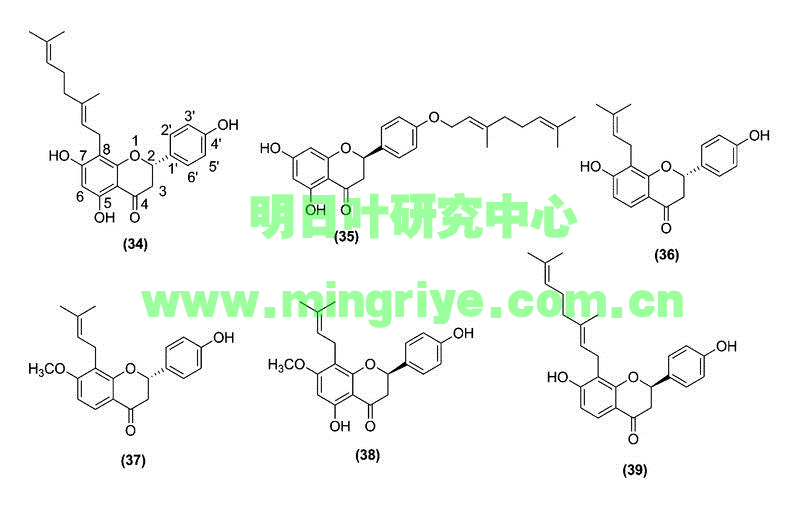
Fig. 3 Structures of bioactive flavanones isolated from A. keiskeiKoidzumi.
Flavanones are distributed throughout the plant kingdom and are found in 42 plant families, both in aerial and belowground tissue. These compounds have been shown to possess radical scavenging, anti-inflammatory, and chemopreventive effects [21]. Flavanones in ashitaba, though less studied than the chalcones 1 and 2, have been studied most for their potential as chemopreventive agents [17].
Other active compounds(其它活性化合物)
Ashitaba also possesses active polyacetylenes, triterpenes, and cyclohexenones. One sesquiterpene, ashitabaol A (40), has been isolated from ashitaba seeds ([Table 1] and [Fig. 4]) and shows free radical scavenging activity [22]. Sesquiterpenes containing a hexahydrobenzofuran or tetrahydro backbone with the 3-methyl-but-2-enylidene unit are extremely uncommon in nature. Compound 40 is only the second reported natural product, after bisbolangelone, with this unusual structure [22].
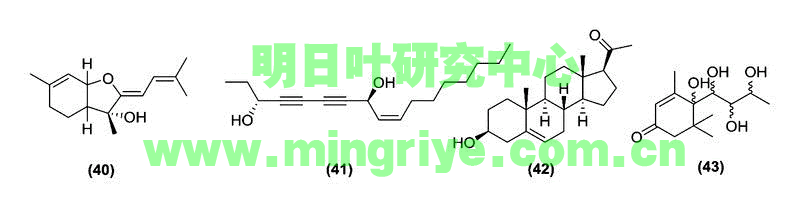
Fig. 4 Other bioactive compounds isolated from A. keiskei Koidzumi.
Biological Activities of Ashitaba
(明日叶的生物活性)
Extracts of ashitaba, whether containing complex mixtures or isolated compounds, are used to treat many diseases. In this section we describe ashitabaʼs cytotoxic, antidiabetic, antiobesity, antioxidant, anti-inflammatory, antithrombotic, antihypertensive, and antimicrobial properties. When possible, structure-activity relationships of known active constituents will be described. A summary of the in vivo and in vitrostudies on ashitaba extracts can be found in [Table 2]. A comprehensive list of known bioactivities for each isolated compound can be found in [Table 3].
Table 2
In vitro and in vivo bioactivity studies on ashitaba extracts.
| Plant part | Extract type | Biological activity tested | Results | References |
| aEdible parts of washed vegetables. b“Ashitaba powder commercially available as a so-called functional food” | ||||
| Cytotoxicity | ||||
| Not specifieda | Ethyl acetate extract | Anticarcinogenicity (in vitro) | Hep G2 cells treated with ashitaba extract (1 mg/mL) showed a 1.42-fold induction of quinone reductase expression, an anticarcinogenic marker enzyme. | [29] |
| Fresh aerial portion | 95 % ethanol extract | Anticarcinogenicity (in vitro) | Murine hepatoma Hepa 1c1c7 cells treated with 25 µg/mL ashitaba extract showed a 2.44-fold induction of NAD(P)H quinone oxidoreductase 1, protecting against quinone-induced damage. | [24] |
| Antidiabetic and antiobesity activity | ||||
| Stem exudate | Ethyl acetate extract | Anti-hyperlipidemic (in vivo) | Male stroke-prone spontaneously hypertensive rats fed a diet containing 0.2 % ashitaba extract for 6 weeks showed increased levels of serum HDL levels and reduced liver triglyceride levels correlated with the downregulation of hepatic acyl-coenzyme A synthetase mRNA. | [54] |
| Leaves and processed products of leaves | Whole leaves, juice, fermented juice, and/or squeeze debris | Anti-adiposity (in vivo) | Male Sprague-Dawley rats fed a high-fat diet with 3–5 % ashitaba whole leaves or a combination of juice and solid squeeze debris for 6 weeks showed decreased liver, kidney, and epididymal fat, and rear fat weights. Ashitaba and its processed products increased luteolin absorption and suppressed diet-induced cholesterol build up in the liver by increasing antioxidant enzyme gene expression. | [55] |
| Stem exudate | Ethyl acetate extract | Anti-adiposity (in vivo) |
Male C57BL/6 mice fed a high-fat diet with 0.01 % ashitaba extract by weight for 16 weeks showed lowered diet-induced body weight and body fat and lowered serum levels of glucose, insulin, and cholesterol when compared to the positive controls. Ashitaba extract regulated lipid metabolism in adipose and liver tissue by activating AMP-activated protein kinase. | [12] |
| Not specifiedb | Ashitaba powder | Anti-adiposity (in vivo) | Male Wistar rats fed a high-fat diet in combination with ashitaba powder at 17, 170, or 1700 mg/100 g body weight for 28 days did not show significant differences in body weight gain, food intake, or relative organ weights when compared to the positive controls. | [56] |
| Dried leaves and stems | Ethanol extract | Antidiabetic (in vivo) | Male Wistar rats fed a high-fructose diet with 3 % ashitaba extract by weight for 11 weeks had 16.5 % lower blood glucose levels, 47.3 % lower serum insulin, 56.4 % lower HOMA-R, and 24.2 % lower triglyceride content, leading to improved insulin resistance and hypertriglyceridemia when compared to the positive controls, likely by enhancing the expression of genes related to the β-oxidation of fatty acids. | [57] |
| Roots | Ethanol extract | Antidiabetic (in vitro) |
Ashitaba extract showed insulin-like activity following incubation with 3 T3-L1 cells. Dose-dependent glucose uptake and differentiation of preadipocytes to adipocytes were observed in treated cells, but not in controls. | [23] |
| Anti-inflammatory activity | ||||
| Root cores, root bark, leaves, and stems | Methanol extract | Xanthine oxidase inhibition (in vitro) | Xanthine oxidase enzyme from bovine serum milk inoculated with 3.12, 6.25, and 12.5 µM of four extracts and 20 mM xanthine was assayed by tracking xanthine oxidation spectrophotometrically. The extracts all showed lower OD273 values than the positive control, allopurinol, indicating that all extracts had potent XO inhibitory activity. Stem and root bark extracts were the most potent inhibitors. | [4] |
| Not specified | n-Hexane extract | Anti-inflammatory (in vitro) | Ashitaba extract 10, 30, 50, or 100 µg/mL suppressed lipopolysaccharide-induced JNK, p38, and ERK1/2 activation in RAW264.7 macrophages. NF-κB was suppressed as well through inhibition of p65 translocation and phosphorylation. | [34] |
| Stem exudate | Yellow exudate, ethyl acetate extract, chalcone-rich, and coumarin-rich fractions | Anti-inflammatory (in vivo) | Male kwl ICR mice (pathogen-free grade) injected intraperitoneally with ashitaba exudate for 7 days before injection with lipopolysaccharide significantly inhibited increase of PAI-1 antigen in lung and liver tissue at 6 and 9 h. Additionally, ethyl acetate extract and chalcone-rich fractions decreased production of LPS-induced PAI-1. | [35] |
| Antihypertensive activity | ||||
| Freeze dried leaves | Purified fraction from 80 % ethanol crude extract | Antihypertensive (in vivo) |
Male spontaneously hypertensive rats given ashitaba extract at 21.8 mg/kg a day for 10 weeks showed significantly lower blood pressure (200 ± 7.3 mmHg) when compared to control rats (211 ± 3.7 mmHg). | [38] |
Table 3 Bioactivities attributed to compounds isolated from ashitaba.
| Compound | Bioactivities | References |
| 1 | Chemopreventive, antidiabetic, anti-adipogenic, anti-inflammatory, antiplatelet, anti-influenza, antibacterial | [4], [12], [17], [18], [23], [24], [25], [26], [27], [30], [32], [33], [36], [37], [39], [40] |
| 2 | Chemopreventive, antidiabetic, anti-adipogenic, anti-inflammatory, antioxidant, antiplatelet, antibacterial | [4], [12], [17], [18], [23], [24], [25], [26], [27], [30], [31], [32], [35], [36], [37], [40] |
| 3 | Anti-inflammatory, antioxidant, antiplatelet, anti-influenza | [4], [31], [35], [36], [39] |
| 4 | Anti-inflammatory | [36] |
| 5 | Antidiabetic, anti-inflammatory, anti-influenza | [18], [33], [35], [39] |
| 6 | Antidiabetic, anti-inflammatory, antiplatelet, | [18], [35], [36] |
| 7 | Chemopreventive, antidiabetic, anti-inflammatory, antioxidant, anti-influenza | [3], [4], [17], [18], [30], [39] |
| 8 | Anti-influenza | [39] |
| 9 | Chemopreventive | [17] |
| 10 | Chemopreventive, anti-inflammatory | [3] |
| 11 | Chemopreventive, anti-inflammatory | [3] |
| 12 | Antidiabetic | [18] |
| 13 | Anti-influenza | [39] |
| 14 | Chemopreventive, anti-inflammatory | [3], [4], [17], [24], [30] |
| 15 | Antidiabetic, antioxidant | [24], [27] |
| 16 | Antidiabetic | [27] |
| 17 | Antidiabetic | [24], [27] |
| 18 | Chemopreventive, antidiabetic | [27] |
| 19 | Antidiabetic | [27] |
| 20 | Antioxidant | [31] |
| 21 | Antioxidant | [31] |
| 22 | Antioxidant | [31] |
| 23 | Chemopreventive | [17] |
| 24 | Chemopreventive; anti-inflammatory | [3], [17] |
| 25 | Antidiabetic | [18] |
| 26 | Chemopreventive, anti-inflammatory | [3], [17] |
| 27 | Chemopreventive | [16] |
| 28 | Chemopreventive, anti-inflammatory | [3], [17] |
| 29 | Chemopreventive, anti-inflammatory | [3], [17] |
| 30 | Chemopreventive, anti-inflammatory | [3], [17] |
| 31 | Chemopreventive, anti-inflammatory | [3], [17] |
| 32 | Antidiabetic | [24] |
| 33 | Anti-inflammatory | [3], [17] |
| 34 | Chemopreventive, anti-inflammatory | [3], [17] |
| 35 | Chemopreventive | [17] |
| 36 | Chemopreventive | [3] |
| 37 | Chemopreventive | [17] |
| 38 | Chemopreventive, anti-inflammatory | [3], [17] |
| 39 | Chemopreventive | [17] |
| 40 | Antioxidant | [22] |
| 41 | Antidiabetic | [24] |
| 42 | Antioxidant | [31] |
| 43 | Antioxidant | [31] |
Antidiabetic and antiobesity activities(抗糖尿病及抗肥胖活性)
Although ashitaba has been purported to possess numerous bioactivities, it has most notably been utilized as a medicinal plant to prevent obesity and its complications. Ashitaba extracts and their isolated constituents have been shown to possess antidiabetic and antiobesity properties. However, the purported properties and modes of action are often contradictory between studies, suggesting a need for more comprehensive analysis of these activities.
Tyrosine-protein phosphatase 1B (PTP1B) negatively regulates the insulin signaling pathway, and is a promising target for the treatment of type II diabetes mellitus [18]. Several compounds isolated from ashitaba, including chalcones 1, 2, 5–7, and 12 and a coumarin (25), inhibited PTP1B activity with IC50values of 0.82–4.42 µg · mL−1. Kinetic studies revealed that compound 12 was a fast-binding competitive inhibitor of PTP1B [18]. Additionally, KK-Ay mice, known to develop hyperglycemia with aging, were fed diets comprised of 0.15 % 1 or 2 and showed suppressed development of insulin resistance as well as lower levels of blood glucose (50 % and 33 % lower, respectively) when compared to controls [23].
Alpha-glucosidases aid in carbohydrate digestion and glucose release, and increased activity of these enzymes can lead to hyperglycemia and the development of type II diabetes. Alpha-glucosidase inhibitors are target molecules for suppressing the onset of this disorder. Four compounds, 2, 14, 32, and 41, had alpha-glucosidase inhibitory activity with IC50 values at or below 20 µM when using 4-nitrophenyl-alpha-D-glucopyranoside as the substrate, considerably lower than the control drug acarbose (IC50 = 384 µM) [24].
To maintain blood sugar homeostasis, it is imperative that skeletal muscle cells uptake glucose. Obesity can impair this uptake and lead to hyperglycemia. The majority of the translocation of glucose is completed by glucose transporter 4 (GLUT4). The activity of GLUT4 is regulated by protein kinase ζ/λ (PKC ζ/λ), protein kinase B (Akt), and adenosine monosphosphate-activated protein kinase (AMPK). The activities of 1 and 2on the activation of GLUT4 glucose translocation in rat skeletal muscle L6 cells were determined and compared to the activity induced by insulin [25]. At 30 µM, 1 stimulated glucose uptake into L6 myotubes 2.8-fold, and 2 stimulated the uptake 1.9-fold, as did insulin. At 10 µM, 1 and 2 induced glucose uptake into L6 myotubes at the same rate as insulin. Of the compounds screened, the prenylated chalcones had the highest GLUT4-inducing activity. The hydrophobic groups may interact directly with the myotubes and facilitate the activation of transporters [25]. Interestingly, the authors found that proteins that typically induce GLUT4 activity, notably PKC ζ/λ, Akt, and AMPK, were not activated by 1 and 2. Thus, 1 and 2 affect other signaling components in the cascade.
The differentiation of adipocytes from preadipocytes plays a large role in the development of obesity [26]. Peroxisome proliferator-activated receptor γ (PPAR-γ) and CCAAT/enhancer-binding proteins (C/EBPs) play important regulatory roles in adipocyte differentiation. Activation of C/EBP-β and C/EBP-δ begins a cascade that increases the expression of C/EBP-α, PPAR-γ, and GLUT4 [26]. AMPK downregulates C/EBP-α and PPAR-γ expression, and modulates the activity of other factors through the inactivation of acetyl-CoA carboxylase (ACC). Inactivation of ACC by phosphorylation halts the biosynthesis of malonyl-CoA, leading to fatty acid oxidation by carnitine palmitoyltransferase-1A (CPT-1A) [12].
Counterintuitively, ligands that activate PPAR-γ have been developed to treat type II diabetes mellitus. Small adipocytes can enhance glucose uptake upon insulin stimulation, enabling the reduction of insulin resistance [23]. One study determined that incubation of 3 T3-L1 cells with compounds 1 and 2 instead of insulin led to equal levels of adipocyte differentiation, but compound 1 resulted in the highest induction of glucose uptake. In a follow-up experiment, the effects of 1 and 2 on PPAR-γ were evaluated, along with the effects of a known PPAR-γ agonist, pioglitazone. Interestingly, only the known agonist pioglitazone activated PPAR-γ, indicating that compounds 1 and 2 induce glucose uptake by a different mechanism than PPAR-γ activation [23].
Other studies have reported contradictory results, and indicate that ashitaba extracts, and particularly compounds 1 and 2, suppress adipocyte differentiation by inactivating PPAR-γ [12], [26]. Treatment of 3T3-L1 cells with 1 and 2 phosphorylated AMPK, leading to its activation and subsequent downregulation of C/EBP-α, C/EBP-β, PPAR-γ, and GLUT4 expression [26]. To determine if adipogenesis was inhibited as a result of AMPK activation, cells were treated with compound C, an AMPK inhibitor, and with compounds 1and 2. Compound C reversed the anti-adipogenic effects of the chalcones, further supporting the involvement of 1 and 2 in AMPK activation [26].
Adiponectin helps to improve insulin resistance, so compounds aiding in adiponectin production may be useful in inhibiting the development of metabolic syndrome [27]. In one study, the effects of compounds 1, 2, and 15–19 were assessed for their effects on adiponectin production in 3T3-L1 adipocytes. All chalcones upregulated the expression of adiponectin mRNA, particularly compounds 17 (7.80-fold induction) and 18(8.27-fold induction). Compounds 1, 2, and 15–19 also significantly enhanced adiponectin production [27].
One clinical study was conducted to determine ashitabaʼs efficacy for treating metabolic syndrome. For this study, 9 subjects ingested ashitaba juice comprised of dried leaves and stems for 8 weeks [28]. Following ingestion, all subjects had significantly lower visceral fat, body fat, and body weight at the end of the 8th week, and no adverse clinical changes were attributed to ashitaba. However, this study lacked controls, and as such provides insufficient evidence for ashitabaʼs efficacy in treating metabolic syndrome.
Numerous in vitro and in vivo studies support the use of ashitaba as an antiobesity and antidiabetic agent, although clinical trials are needed to confirm the relevance of these compounds in humans. However, contradictions in the literature suggest that further research to understand the mechanisms of action and molecular targets of active constituents should be conducted in addition to clinical tests. Additionally, research on other ashitaba constituents besides compounds 1 and 2 may lead to novel discoveries.
Chemopreventive activity(化学预防活性)
Ashitaba extracts have been shown to possess chemopreventive properties in vitro, involving both antiproliferative and antimutagenic mechanisms. Quinone reductase plays an important role in detoxification by reducing electrophilic quinones. This defends cells against quinone-induced cytotoxic effects and subsequent carcinogenesis [29]. An ethyl acetate-soluble crude vegetable extract of ashitaba was shown to induce Hep G2 cell quinone reductase activity by nearly 50 % in 48 h (1.42 ± 0.06-fold induction) [29]. Unfortunately, the part of the plant extracted was not specified, and chemical consituents were not determined [29]. Another study determined that NAD(P)H quinone oxidoreductase 1 (NQO1), which also protects against quinone-induced damage, was activated in murine hepatoma Hepa 1c1c7 cells by an ethanol-soluble extract of ashitaba (2.44-fold induction at 25 µg · mL−1). Subsequent compound isolation indicated that four chalcones, 1, 2, 14, and 18, had the highest rates of NQO1 induction when tested against murine hepatoma Hepa 1c1c7 cells [24].
Several researchers have studied the inhibitory effects of ashitaba compounds on the induction of the Epstein-Barr virus Early Antigen (EBV-EA) by 12-O-tetradecanoyl phorbol 13-acetate (TPA). EBV is associated with numerous diseases, including types of lymphoma and cancer, and the inhibitory effects on its induction are often used to evaluate antitumor-promoting activity in preliminary studies [3], [17], [30]. In Raji cells, compounds 10, 11, 14, 30, 34, 36, and 38 were more potent inhibitors than retinoic acid, the reference compound, with IC50 values ranging from 215–320 mol ratio 32 pmol−1 TPA [3]. In a previous study, compounds 1, 2, 7, 9, 23, 24, 26, 28, 29, 31, 35, 37, and 39 also showed potent inhibitor effects, ranging from 92–100 % inhibition at 1000 mol ratio, and 51–84 % at 500 mol ratio. In Raji cells, inhibitors 1, 14, 35, 37, and 39 were more potent than the reference compound β-carotene [17]. Compound 27 was also found to have TPA-inhibiting properties [16]. All of these active compounds have, in addition to the chalcone, coumarin, or flavanone backbone, a prenyl or genanyl group, suggesting that the addition of isoprene units results in an increase in chemopreventive potential [3].
Three prenylated chalcones, 1, 2, and 7, were transformed by the fungal microbe Aspergillus satoi, resulting in flavanone, prenyl chain hydrated, and ring-B-hydroxylated derivatives. Several flavanone and prenyl chain derivatives, along with compounds 1, 2, and 7, also suppressed EBV-EA induction in Raji cells with IC50 values ranging from 211–348 mol ratio 32 pmol−1 TPA [30]. Interestingly, biotransformation products in which the prenyl or geranyl chain was hydrated had the most potent inhibitor effects, even more than parent compounds. Products that had been cyclized from chalcones to flavanones, on the other hand, showed weakened activity [30].
A prenyl chain hydrated biotransformation product of 1, 2″,3″-dihydro-4,3″-dihydroxyderricin (44; [Fig. 5]), was shown to possess cytotoxic activity (IC50 = 2.9 µM) against human leukemia cells (HL60) [30]. To determine if this compound played a role in regulating apoptosis, a follow-up experiment was conducted. Indeed, HL60 cells treated with 30, 40, or 50 µM of this compound displayed morphological characteristics consistent with apoptosis, including chromatin condensation, nuclei fragmentation, and mitochondrial membrane collapse [30]. In two-stage carcinogenesis tests in mouse skin, it was determined that 14 and 6″,7″-dihydro-7″-hydroxyxanthoangelol F (45; [Fig. 5]), a hydrated prenyl chain biotransformation product of 7, inhibited the rate and number of skin tumors produced in mice. When topically treated twice a week with 7,12-dimethylbenz[a]anthracene (DMBA) and TPA, control mice developed papillomas 100 % by 11 weeks. When treated topically with 85 nmol of 45 before application of DMBA and TPA, the incidence was lowered to 27 % at 11 weeks and 87 % at 20 weeks [30]. Similarly, after 10 weeks, only 20 % of mice given a topical treatment (85 nmol) of 14 before contact with tumor-inducing compounds developed papillomas when compared to 100 % of controls. At 20 weeks, 87 % of treated mice had developed papillomas [3].

Fig. 5 Bioactive chalcone biotransformation products from A. keiskeiKoidzumi.
Several in vitro studies have been conducted on ashitabaʼs cytotoxic effects. However, only a few in vivotests have been completed using animal models, and no clinical trials have been conducted in humans. As such, no conclusive evidence yet exists to confirm the use of ashitaba compounds as anticancer agents. More robust animal studies followed by clinical trials are necessary to support the use of these constituents for cancer treatment.
Oxidative stress relief and anti-inflammatory activity(缓解氧化应激和抗炎活性)
Compounds isolated from ashitaba have been shown to possess antioxidant properties, thereby reducing inflammation by a number of routes. Modes of action include xanthine oxidase (XO) inhibition [4], free-radical scavenging activity [22], [24], [31], and reduction in expression of proinflammatory transcription factors [32], [33], [34].
XOreduces molecular oxygen, leading to anionic O2 − and hydrogen peroxide. These free radicals commonly result in inflammation, so regulators of XO activity could be potent anti-inflammatory agents [4]. When tested against XO from bovine serum milk, ashitaba stem and root bark extracts demonstrated significant XO regulation, as indicated by increased levels of xanthine oxidation. Isolated chalcones 1, 2, 3, 7, and 14showed IC50 values against XO ranging from 8.1 to 54.3 µM. Compound 2 was found to be the most effective (IC50 = 8.1 µM), and likely functions as a reversible inhibitor of XO [4].
Generation of free radicals can result in damage to cellular machinery. Compound 40 from ashitaba seed coat tissue exhibited 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) free radical scavenging activity [22]. Additionally, compounds 2, 15, 42, and 43 were found to scavenge 2,2,-diphenyl-1-picrylhadrazyl (DPPH) radicals [24], indicating that these compounds may be useful antioxidant agents. Compounds 3 and 20–22 were also shown to scavenge superoxide radicals (0.51–1.1 µM IC50 values), with 20 showing the most potent activity [31].
Nitric oxide (NO) is another mutagen that affects microbial and mammalian cells due to the production of free radicals. When tested against Chang liver cells, compounds 7, 10, 11, 14, 24, 26, 28–31, 33, 34, and 38 showed inhibitory effects on NO almost equal to the reference compound glyzyrrhizin [3], [17]. In another study, compounds 1 and 2 were also shown to suppress the production of NO in RAW264 macrophages, with negligible effects on cellular function [32]. The authors noted that prenylated chalcones were more effective in suppressing NO formation, with 2 being more potent than 1. Since 2 contains a geranyl group and 1 contains a dimethylallyl group, it is possible that the increased hydrophobicity of additional isoprene units facilitates compound accumulation into the cell, promoting antioxidative activity [32].
Tumor necrosis factor alpha (TNF-α) has been implicated as an important participant in the induction of inflammation [32] and is regulated by transcription factors activator protein 1 (AP-1) and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Ashitaba extract and compound 2 were shown to inhibit inflammation induced by TNF-α in male kwl ICR mice [35]. Another study determined that isolated compounds 1 and 2 had similar TNF-α suppressing effects in RAW264 macrophages [32], and compound 5induced suppression in porcine aortic endothelial cells [33]. In RAW246.7 macrophages, the n-hexane ashitaba extract had anti-inflammatory activity resulting from the downregulation of NF-κB-dependent gene products [34]. Ashitabaʼs anti-inflammatory properties can also be attributed to its effects on histamine release. Histamine is an important messenger compound released by mast cells in response to foreign agents and, consequently, plays a large role in allergic reactions and inflammation. Compounds 1–4 and 6have been illustrated to show histamine release inhibition in rat peritoneal mast cells [36].
Again, many tests have been conducted in vitro on ashitaba constituents and their antioxidant and anti-inflammatory effects, but the translatability of these tests to in vivo and clinical tests has yet to be determined. Additionally, it should be noted that most substances exhibit some antioxidant effects, especially at high enough concentrations, and calorimetric tests such as those used to evaluate DPPH scavenging activity do not provide strong enough data to confirm antioxidant activity. More robust analyses utilizing cell lines are less likely to yield false positive results and are thus provide more valuable indications of antioxidant capacity.
Antithrombotic activity(抗血栓活性)
Compounds isolated from ashitaba stem tissue show promise as antithrombotic agents due to their antiplatelet activity. Increased levels of plasminogen activator inhibitor-1 (PAI-1) can result in persistent blood clots leading to thrombotic complications, including heart attacks and strokes. TNF-α, a player in inflammation responses, is also involved in the induction of PAI-1 expression. Again, chalcones in ashitaba, namely compounds 2, 3, 5, and 6, were found to suppress activities induced by TNF-α, resulting in a reversal of PAI-1 increase in human umbilical vein endothelial cells [35].
In another study, 1 and 2 illustrated dose-dependent antiplatelet activity against a number of platelet aggregation inducers, including collagen-, phorbol 12-myristate 13-acetate (PMA), and platelet-activating factor (PAF) in washed rabbit platelets [37]. The authors found that 1 and 2 have antiplatelet activity equivalent to aspirin. Because 1 and 2 did not show strong inhibition against thrombin-induced clotting, which is induced through the phospholipase C-β (PLC-β) pathway, the authors concluded that the activity results through the intracellular mobilization of Ca2+ by the phospholipase-γ (PLC-γ) pathway, which is also stimulated by collagen and PAF [37].
Blood pressure-reducing activity
Although little research has been completed regarding the antihypertensive properties of ashitaba, preliminary research has shown promise for its use in reducing blood pressure. The renin-angiotensin (R-A) system involves the angiotensin I-converting enzyme (ACE), which produces angiotensin II, a vasoconstrictor [38]. ACE is a major player in essential hypertension, which is the most prominent type of hypertension diagnosed in the medical field. A compound isolated from ashitaba leaf tissue was found to inhibit ACE from rabbit lung acetone powder. It showed no effect on body weight or serum lipid levels in spontaneously hypertensive rats [38]. Mass spectral data and inhibitory activity data suggested that this compound may be structurally related to nicotianamine. More data is required, both in vitro and in vivo, to determine the efficacy of ashitaba in treating hypertension.
Antimicrobial activity(抗微生物活性)
Ashitaba chalcones have also shown promise as antimicrobial agents. For example, compounds 1, 3, 5, 7, 8, and 13 were found to have potent influenza virus neuraminidase (NA) inhibition on recombinant NA from the 1918 Spanish flu virus (A/Bervig_Mission/1/18), suggesting that they may be useful as anti-influenza agents [39]. The authors noted that the activity against NA was influenced by small changes in molecular structure. Elongation of prenyl chains from dimethylallyl groups to geranyl groups caused a 2-fold loss of activity. When 2-hydroxy-3-methyl-3-butenyl alkyl (HMB) groups were also prenylated, a 2-fold loss of activity was also observed. Conversion of dimethylallyl and geranyl groups to their HMB counterparts, on the other hand, resulted in a gain of activity [39]. Compound 5 was found to be the most potent inhibitory agent, and the authors suggested that the location of the HMB group may be responsible for its potency [39].
Compounds 1 and 2 have also been identified as potent antibacterial agents, particularly against gram-positive bacteria. Using an agar dilution test, these chalcones were shown to have MIC values below 7 µg · mL−1 for Staphylococcus aureus 209-P, and below 2 µg · mL−1 against Bacillus subtilis PCI-219, B. subtilisATCC_6633, Bacillus cereus FDA-5, S. aureus IFO-3060, Staphylococcus epidermidis IFO-3762, andMicrococcus luteus IFO-12708 [40]. These compounds were also shown to have potent antibacterial activity (MIC ≤ 1.00 µg · mL−1) against plant pathogenic bacteria, including Agrobacterium tumefaciens IFO-3058,Pseudomonas syringae pv. phaseolicola IFO-12656, Pseudomonas syringae pv. tabaci IFO-3508, Pseudomonas stutzeri IFO-12510 [40].
Bioavailability(生物药效率)
Ashitaba chalcones possess a number of purported health effects, but no reports about the bioavailability of its prenylated chalcones in human tissue currently exist. However, several studies have examined the pharmacokinetic properties of xanthohumol, a prenylated chalcone found in hops, in both humans and rats. Rats and humans given oral administrations of hops typically had nanomolar concentrations of xanthohumol and related prenylflavonoids in their plasma [41], [42], [43]. In a study conducted on human microbiota-associated rats, the overall excretion of xanthohumol and its related metabolites after two days was only 4.2 % of the ingested amount, indicating that this compound is likely hydrolyzed by human intestinal microorganisms [43]. Additionally, interindividual variability in gut microbiota was found to play a large role in the availability of xanthohumol, and some species of bacteria rapidly hydrolyze this chalcone into 8-prenylnaringenin, a potent phytoestrogen that can affect estrogen signaling pathways [42], [43], [44]. The associated health effects of the consumption of xanthohumol depends largely on the amount ingested as well as on the phenotype of the individual ingesting this compound. Whether or not these trends will translate to other prenylated chalcones such as those contained in ashitaba tissue is uncertain, and future studies should aim to determine the bioavailability of these compounds. Additionally, studies determined to identify the in vivo differences in metabolism in individuals with variable gut microbiota should be conducted.
Toxicology(毒理)
The safety of ashitaba was assessed using multiple good laboratory practice (GLP) tests, including a bacterial reverse mutation test, chromosome aberration test, in vivo mouse micronucleus test, acute oral toxicity tests, and a 13-week oral toxicity test [45]. Additionally, the safety of using ashitaba for cosmetic purposes was assessed using the eye irritancy test [46].
Ashitaba yellow sap chalcone powder was found to be non-mutagenic based on results from the bacterial reverse mutation assay, chromosome aberration assay, and in vivo micronucleus assay. Decreased platelet counts were noted in male and female Sprague-Dawley rats, which is an expected effect based on known antithrombotic properties of several bioactive chalcones. It was noted that the magnitude of the platelet count reduction is marginal, and not of toxicological significance without other clinical signs [45]. Statistically significant levels of serum alkaline phosphatase, total cholesterol, and serum phospholipid and triglycerides were noted in rats fed the highest amount of ashitaba chalcone powder (1000 mg · kg−1 body weight). This is also an unsurprising discovery based on the known effects of ashitaba on cholesterol transport and lipid metabolism.
Interestingly, male and female rats fed the highest dose showed dilated intestinal lacteals involved in the absorption of dietary fats in the small intestine. Such dilation is indicative of lymphangiectasia, a rare disorder that can lead to edema and its related complications, including fatigue, abdominal pain, diarrhea, vitamin deficiencies, and weight loss [47]. The observation of jejunal lacteal dilation is extremely rare in rodent toxicity studies, so the no observed adverse effect level (NOAEL) of ashitaba powder was concluded to be 300 mg · kg−1 body weight [45].
To determine the safety of ashitaba as a topical agent, 100 mg of aqueous or ethanol ashitaba leaf extracts were dropped into the eyes of New Zealand White rabbits and the reactions were assessed each day for 7 days. No damages were reported in terms of corneal lesions, turbidity, or eyelid swelling [46]. As such, aqueous and ethanol extracts of ashitaba are candidates for use as cosmetic agents.
Although the issue of furanocoumarin toxicity has not been specifically addressed with ashitaba, it should be noted that a number of furanocoumarins have been shown to be phototoxic and photogenotoxic in addition to interfering with drug metabolism by cytochrome P450 enzymes [48]. Ashitaba, as is typical with members of the Apiaceae family, contains bioactive furanocoumarins (25) and dihydrofuranocoumarin analogs (23, 27). In fact, compound 25 has illustrated phototoxic and photogenotoxic effects in a number of studies [48], [49]. An assessment by the Senate Commission on Food Safety reported that compound 25and its isomer 8-methoxypsoralen are only weakly mutagenic in the absence of UV light, but in the presence of UV radiation, these compounds bind covalently to DNA in bacteria and yeasts, leading to genotoxic and mutagenic effects [49]. Because numerous coumarin derivatives are present within ashitaba plant tissue, it is necessary to test individual compounds for phototoxic and photogenotoxic effects. Additionally, bioavailability is affected both by extract composition as well as the route of administration, and studies are required to determine if phototoxic compounds, such as compound 25, are at high enough concentrations to be of toxicological concern.
The toxicological data on ashitaba extracts has been addressed to some extent, but more robust toxicological examinations, such as teratogenicity tests, are needed. Additionally, toxicological analyses on isolated compounds should be conducted. In particular, the toxicological profiles of prenylated chalcones (1–22), the representative structural class of ashitaba, as well as those of furanocoumarins (23, 25, 27), must be thoroughly characterized to determine ranges of toxicity.
Conclusions
(结论)
This review summarizes the known phytochemistry and bioactivities of ashitaba. Although there is some inconsistency in the literature, most notably on the effect of ashitaba on adipocyte differentiation, in vivoevidence supports the use of ashitaba as a medicinal plant with antiobesity properties. Thorough in vitrotesting has been completed for many of ashitabaʼs other purported bioactivities, but more robust in vivoand clinical experiments are needed to confirm the medicinal applications from a clinical standpoint. In particular, clinical testing is warranted to assess ashitabaʼs antidiabetic and antiobesity efficacy, and more preclinical data is needed before pursuing clinical trials of other biological activities. Future studies should focus not only on the most abundant chalcones 1 and 2, but also on the bioactivities of other related compounds found in ashitaba.
Acknowledgments
(感谢)
Dr. Nicholas Oberlies is acknowledged for his guidance with this project.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
(引用)
1 Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the Use of complementary Health approaches among Adults: United States, 2002 – 2012. National Health Statistics Report, No. 79. Hyattsville, MD: National Center for Health Statistics; 2015
2 Sarkar SD, Nahar L. Natural medicine: the genus Angelica . Curr Med Chem 2004; 11: 1479-1500
3 Akihisa T, Tokuda H, Hasegawa D, Ukiya M, Kimura Y, Enjo F, Suzuki T, Nishino H. Chalcones and other compounds from the exudates of Angelica keiskei and their cancer chemopreventive effects. J Nat Prod 2006; 29: 38-42
4 Kim DW, Curtis-Long MJ, Yuk HR, Wang Y, Song YH, Jeong SH, Park KH. Quantitative analysis of phenolic metabolites from different parts of Angelica keiskei by HPLC-ESI MS/MS and their xanthine oxidase inhibition. Food Chem 2014; 153: 20-27
5 Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 2011; 126: 485-493
6 Zhang H, Liu JJ, Sun J, Yang XH, Zhao TT, Lu X, Gong HB, Zhu HL. Design, synthesis, and biological evaluation of novel chalcone derivatives as antitubulin agents. Bioorg Med Chem 2012; 20: 3212-3218
7 Mahapatra DK, Asati V, Bharti SK. Chalcones and their therapeutic targets for the management of diabetes: structural and pharmacological perspectives. Eur J Med Chem 2015; 92: 839-865
8 Battenberg OA, Yang Y, Verhelst SH, Sieber SA. Target profiling of 4-hydroxyderricin in S. aureus reveals seryl-tRNA synthetase binding and inhibition by covalent modification. Mol Biosyst 2013; 9: 343-351
9 Yadav VR, Prasad S, Sung B, Aggarwal BB. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int Immunopharmacol 2011; 11: 295-309
10 Ceremuga TE, Johnson LA, Adams-Henderson J, McCall S, Johnson D. Investigation of the anxiolytic effects of xanthohumol, a component of Humulus lupulus (Hops), in the male Sprague-Dawley rat. AANA J 2013; 81: 193-198
11 Zhou B, Xing C. Diverse molecular targets for chalcones with varied bioactivities. Med Chem 2015; 5: 388-404
12 Zhang T, Yamashita Y, Yasuda M, Yamamoto N, Ashida H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food Funct 2015; 6: 135-145
13 Bourgaud F, Hehn A, Larbat R, Doerper S, Gontier E, Kellner S, Matern U. Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem Rev 2006; 5: 293-308
14 Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des 2004; 10: 3813-3833
15 Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem 2008; 15: 2664-2679
16 Okuyama T, Takata M, Takayasu J, Hasegawa T, Tokuda H, Nishino A, Nishino H, Iwashima A. Anti-tumor-promotion by principles obtained from Angelica keiskei . Planta Med 1991; 57: 242-246
17 Akihisa T, Tokuda H, Ukiya M, Iizuka M, Schneider S, Ogasawara K, Mukainaka T, Iwatsuki K, Suzuki T, Nishino H. Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett 2003; 201: 133-137
18 Li J, Gao L, Meng F, Tang CL, Zhang RJ, Li JY, Luo C, Li J, Zhao WM. PTP1B inhibitors from stems ofAngelica keiskei (Ashitaba). Bioorg Med Chem Lett 2015; 25: 2028-2032
19 Ogawa H, Nakamura R, Baba K. Beneficial effect of laserpitin, a coumarin compound from Angelica keiskei,on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 2005; 32: 1104-1109
20 Wong E. The role of chalcones and flavanones in flavonoid biosynthesis. Phytochemistry 1968; 7: 1751-1758
21 Khan MK, Zill EH, Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J Food Comp Anal 2014; 33: 85-104
22 Aoki N, Ohta S. Ashitabaol A, a new antioxidative sesquiterpenoid from seeds of Angelica keiskei . Tetrahedron Lett 2010; 51: 3449-3450
23 Enoki T, Ohnogi H, Nagamine K, Kudo Y, Sugiyama K, Tanabe M, Kobayashi K, Sagawa H, Kato I.Antidiabetic activities of chalcones isolated from a Japanese herb, Angelica keiskei . J Agric Food Chem 2007; 55: 6013-6017
24 Luo L, Wang R, Wang X, Ma Z, Li N. Compounds from Angelica keiskei with NQO1 induction, DPPH scavenging and α-glucosidase inhibitory activities. Food Chem 2012; 131: 992-998
25 Kawabata K, Sawada K, Ikeda K, Fukuda I, Kawasaki K, Yamamoto N, Ashida H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol Nutr Food Res 2011; 55: 467-475
26 Zhang T, Sawada K, Yamamoto N, Ashida H. 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadipocytes to adipocytes via AMPK and MAPK pathways. Mol Nutr Food Res 2013; 57: 1729-1740
27 Ohnogi H, Kudo Y, Tahara K, Sugiyama K, Enoki T, Hayami S, Sagawa H, Tanimura Y, Aoi W, Naito Y, Kato I, Yoshikawa T. Six new chalcones from Angelica keiskei inducing adiponectin production in 3T3-L1 adipocytes. Biosci Biotechnol Biochem 2012; 76: 961-966
28 Ohnogi H, Hayami S, Kudo Y, Enoki T. Efficacy and safety of ashitaba (Angelica keiskei) on the patients and candidates with metabolic syndrome: a pilot study. JJCAM 2012; 9: 49-55
29 Hashimoto K, Kawamata S, Usui N, Tanaka A, Uda Y. In vitro induction of the anticarcinogenic marker enzyme, quinone reductase, in human hepatoma cells by food extracts. Cancer Lett 2002; 180: 1-5
30 Akihisa T, Motoi T, Seki A, Kikuchi T, Fukatsu M, Tokuda H, Suzuki N, Kimura Y. Cytotoxic activities and anti-tumor-promoting effects of microbial transformation products of prenylated chalcones from Angelica keiskei . Chem Biodivers 2012; 9: 318-330
31 Aoki N, Muko M, Ohta E, Ohta S. C-Geranylated chalcones from the stems of Angelica keiskei with superoxide-scavenging activity. J Nat Prod 2008; 71: 1308-1310
32 Yasuda M, Kawabata K, Miyashita M, Okumura M, Yamamoto N, Takahashi M, Ashida H, Ohigashi H.Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J Agric Food Chem 2014; 62: 462-467
33 Sugii M, Ohkita M, Taniguchi M, Baba K, Kawai Y, Tahara C, Takaoka M, Matsumura Y. Xanthoangelol D isolated from the roots of Angelica keiskei inhibits endothelin-1 production through the suppression of nuclear factor kappaB. Biol Pharm Bull 2005; 28: 607-610
34 Lee HJ, Choi TW, Kim HJ, Nam D, Jung SH, Lee EH, Lee HJ, Shin EM, Jang HJ, Ahn KS, Shim BS, Choi SH, Kim SH, Sethi G, Ahn KS. Anti-inflammatory activity of Angelica keiskei through suppression of mitogen-activated protein kinases and nuclear factor κB activation pathways. J Med Food 2010; 13: 691-699
35 Ohkura N, Nakakuki Y, Taniguchi M, Kanai S, Nakayama A, Ohnishi K, Sakata T, Nohira T, Matsuda J, Baba K, Atsumi G. Xanthoangelols isolated from Angelica keiskei inhibit inflammatory-induced plasminogen activator inhibitor 1 (PAI-1) production. Biofactors 2011; 37: 455-461
36 Nakata K, Baba K. Histamine release inhibition activity of Angelica keiskei . Nat Med 2001; 55: 32-34
37 Son DJ, Park YO, Yu C, Lee SE, Park YH. Bioassay-guided isolation and identification of anti-platelet-active compounds from the root of Ashitaba (Angelica keiskei Koidz.). Nat Prod Res 2014; 28: 2312-2316
38 Shimizu E, Hayashi A, Takahashi R, Aoyagi Y, Murakami T, Kimoto K. Effects of angiotensin I-converting enzyme inhibitor from ashitaba (Angelica keiskei) on blood pressure of spontaneously hypertensive rats. J Nutr Sci Vitaminol 1999; 45: 375-383
39 Park JY, Jeong HJ, Kim YM, Park SJ, Rho MC, Park KH, Ryu YB, Lee WS. Characteristic of alkylated chalcones from Angelica keiskei on influenza virus neuraminidase inhibition. Bioorg Med Chem Lett 2011; 21: 5602-5604
40 Inamori K, Baba K, Tsujibo H, Taniguchi M, Nakata K, Kozawa M. Antibacterial activity of two chalcones, xanthoangelol and 4-hydroxyderricin, isolated form the root of Angelica keiskei KOIDZUMI. Chem Pharm Bull 1991; 39: 1604-1605
41 Wesolowska O, Gasiorowska J, Petrus J, Czarnik-Matusewicz B, Michalak K. Interaction of prenylated chacones and flavaonones from common hop with phosphatidylcholine model membranes. Biochim Biophys Acta 2014; 1838: 173-184
42 Bolca S, Li J, Nikolic D, Roche N, Blondeel P, Possemiers S, De Keukeleire D, Bracke M, Heyerick A, van Breemen RB, Depypere H. Disposition of hop prenylflavnoids in human breast tissue. Mol Nutr Food Res 2010; 54 (Suppl. 02) S284-S294
43 Hanske L, Loh G, Sczensky S, Blaut M, Braune A. Recovery and metabolism of xanthohumol in germ-free and human microbiota-associated rats. Mol Nutr Food Res 2010; 52: 1405-1413
44 Possemiers S, Heyerick A, Robbens V, De Keukeleire D, Verstraete W. Activation of proestrogens from hops (Humulus lupulus) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J Agric Food Chem 2005; 53: 6281-6288
45 Maronpot RR. Toxicological assessment of Ashitaba Chalcone. Food Chem Toxicol 2015; 77: 111-119
46 Son H, Yoon E, Cha Y, Kim MA, Shin YK, Kim JM, Choi YH, Lee SH. Comparison of the toxicity of aqueous and ethanol fractions of Angelica keiskei leaf using the eye irritancy test. Exp Ther Med 2012; 4: 820-824
47 Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmannʼs disease). Orphanet J Rare Dis 2008; 3: 1-8
48 Messer A, Raquet N, Lohr C, Schrenk D. Major furocoumarins in grapefruit juice II: phototoxicity, photogenotoxicity, and inhibitory potency vs. cytochrome P450 3A4 activity. Food Chem Toxicol 2012; 50: 756-760
49 Eisenbrand G. Toxicological assessment of furocoumarins in foodstuffs, opinion of the Senate Commission on Food Safety of the German Research Foundation–shortened version. Mol Nutr Food Res 2007; 51: 367-373
50 Kozawa M, Morita N, Baba K, Hata K. The structure of xanthoangelol, a new chalcone from the roots ofAngelica keiskei Koidzumi (Umbelliferae). Chem Pharm Bull 1977; 25: 515-516
51 Baba K, Nakata K, Taniguchi M, Kido T, Kozawa M. Chalcones from Angelica keiskei . Phytochemistry 1990; 29: 3907-3910
52 Nakata K, Taniguchi K, Baba K. Three chalcones from Angelica keiskei . Nat Med 1999; 53: 329-332
53 Baba K, Kido T, Yoneda Y, Taniguchi M, Kozawa M. Chemical components of Angelica keiskei Koidzumi v. components of the fruits and comparison of coumarins and chalcones in the fruits roots and leaves. Shoyakugaku Zasshi 1990; 44: 235-239
54 Ogawa H, Nakashima S, Baba K. Effects of dietary Angelica keiskei on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 2003; 30: 284-288
55 Kim E, Choi J, Yeo I. The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet. Nut Res Pract 2012; 6: 9-15
56 Nagata J, Morino T, Saito M. Effects of dietary Angelica keiskei on serum and liver lipid profiles, and body fat accumulations in rats. J Nutr Sci Vitaminol 2007; 53: 133-137
57 Ohnogi H, Hayami S, Kudo Y, Deguchi S, Mizutani S, Enoki T, Tanimura Y, Aoi W, Naito Y, Kato I, Yoshikawa T. Angelica keiskei extract improves insulin resistance and hypertriglyceridemia in rats fed a high-fructose drink. Biosci Biotechnol Biochem 2012; 96: 928-932
Correspondence
Nadja B. Cech
Department of Chemistry and Biochemistry
University of North Carolina at Greensboro
435 Patricia A. Sullivan Science Building
PO Box 26170
Greensboro NC 27402
USA
*AD:购买明日叶茶、明日叶粉,十年品牌,品质护航,添加微信:156440577









明日叶具有很好的抗糖尿病、抗高血压、抗氧化功效,值得大力推广。